Which Element Has the Smallest First Ionization Energy
Electronegativity increases moving from the bottom left-hand corner of the periodic table toward the upper right-hand corner. The energy change for the reaction Hgl Hgg e- is therefore A 1006 kJmol.
3 3 Trends In Ionization Energy Chemistry Libretexts
Rubidium has lowest ionization energy this is beacuse when we go down the group in alkali metal group electrons gets added to new shells.
. 58 - Ionization energy. The easier the atom can release the electron the lower the energy required to remove it ie. Which element has the smallest second ionization energy.
B HCN O2 CO2 c H2O CO C2. First ionization energy is the energy required to separate one valence electron from an atom in gas phase. Francium Fr francium has the lowest ionization energy.
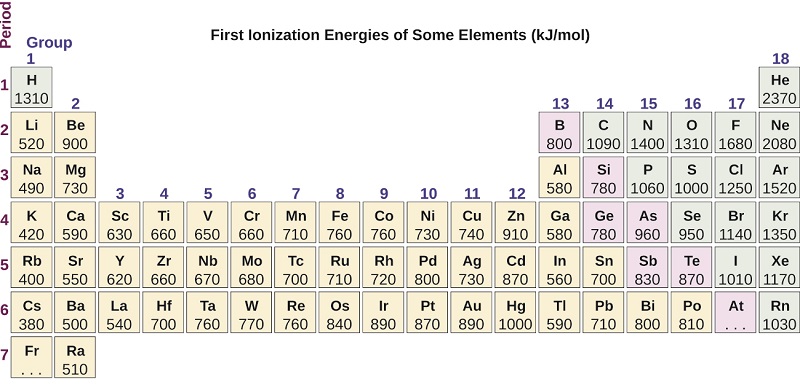
The lower the first ionization energy. 6 BF3 Cl2 O2 10. Helium has the highest first ionization energy and francium has the lowest first ionization energy.
59 - Elements in human body. H The unit of ionization energy is. Which element has the smallest ionization energy.
Which element has the smallest first ionization energy. The first ionization energy of mercury is 1006 kJmol. The element with the lowest ionization energy is cesium Cs.
Helium Thus helium has the largest first ionization energy while francium has one of the lowest. Answer and Explanation. A Rb B Mg C I D As E F Select the element with the greatest metallic character.
The the other elements are in upper periods than Rubidium. Which element has the smallest second ionization energy. What element has the smallest first ionization energy.
Potassium option C has the smallest first ionization energy from the given elements. Answer 1 of 2. Which of the following sets contain all linear molecules.
An elements second ionization energy is the energy required to remove the outermost or least bound electron from a 1 ion of the element. Prior to its discovery it was referred to as eka-caesium. Which of these elements has the smallest first ionization energy.
A Cs b Ga c K d Bi e As 8. This result in less attraction towards last shell electrons and results in lower ionization energy. Cesium has smallest ionization energy.
A H2S HCN CO2. Which of the following elements has the smallest first ionization energy. The ionization energy is minimum for cesium maximum for fluorine.
Which element has the highest first ionization energy. For chemistry students and teachers. Fr francium has the lowest ionization energyFr franciumfranciumFrancium is a chemical element with the symbol Fr and atomic number 87.
Thus helium has the largest first ionization energy. The first chemical element is Cesium and the last one is Helium. A Mg b Li c S d O e Ca 9.
The element with the lowest electronegativity value is francium which has an electronegativity of 07. The first ionization energy of boron is less than that of beryllium and the first. Prior to its discovery it was referred to as eka-caesium.
As By signing up youll get thousands of. C less than 1006 kJmol. Cesium has atomic number 55 and is in the fifth row of the periodic table.
The ionization energy decreases from top to bottom in groups and increases from left to right across a period. The lower the first ionization energy. Potassium option C has the smallest first ionization energy from the given.
Lithium has the highest second ionization energy. D H2S CO CO2. A Cs b Ga cK d Bi e As 8.
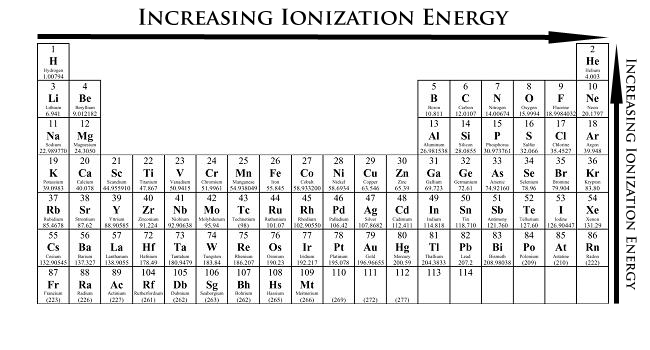
The first ionization energy varies in a predictable way across the periodic table. Which element has the smallest first ionization energy. 119 rows - Elements in earthcrust.
Potassium option C has the smallest first ionization energy from the given elements. Ionization energy is the energy that an atom at ground state must be absorb to release an electron to form a cation. D H2S CO CO2.
E is equal to the second ionization energy of mercury. Rb Rubidium is correct answer. The first ionization energy of boron is smaller than beryllium and the first ionization energy of oxygen is smaller than nitrogen.
4 HyS HCN CO2 6 HCN O2 CO2 c H2O CO Cl2. This value uses the Pauling scale to measure electronegativity. Which of the following sets contain all linear molecules.
4 rows Correspondingly which element has the smallest first ionization energy. Because positive charge binds electrons more strongly the second ionization energy. Its radius is large and there is only in one electron in last.
60 - Covalenz radius. D is equal to the electron affinity of mercury. First ionization energy is the energy required to separate one valence electron from an atom in gas phase.
Based on the graph rank the group 2A elements in periods 1-5 in decreasing order of first ionization energy. Beryllium magnesium calcium strontium How is a jump in ionization energy related to the valence electrons of the element. B greater than 1006 kJmol.
The easier the atom can release the electron the lower the energy required to remove it ie. These observations can be explained by looking at the electron configurations of these elements. A Ne B Rb C P D I E Cl Define what is meant by ionization energy and write a balanced.
A Mg b Li c S d O е Са 9. The tabular chart on the right is arranged by Ionization energy.

Q Solved Which Element Requires The Least Amount Of Energy To Remove The Most Loosely Held Electron From A Gaseous Atom In The Ground State

Solved Element Has The Lowest Ionization Energy

Sulfur Element Facts Properties Production Uses Study Chemistry Electron Configuration Ionization Energy
How Would You Arrange The Following Elements In Order Of Increasing Ionization Energy Te Pb Cl S Sn Socratic

Periodic Variations In Element Properties Chem 1305 Introductory Chemistry
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic

The Parts Of The Periodic Table
What Is The Order Of The Ionization Energy Of O S Br And I Quora
Carbon Group Element Chemical Elements Britannica
Which Atom Has The Smaller Ionization Energy Be Or Mg Why Quora
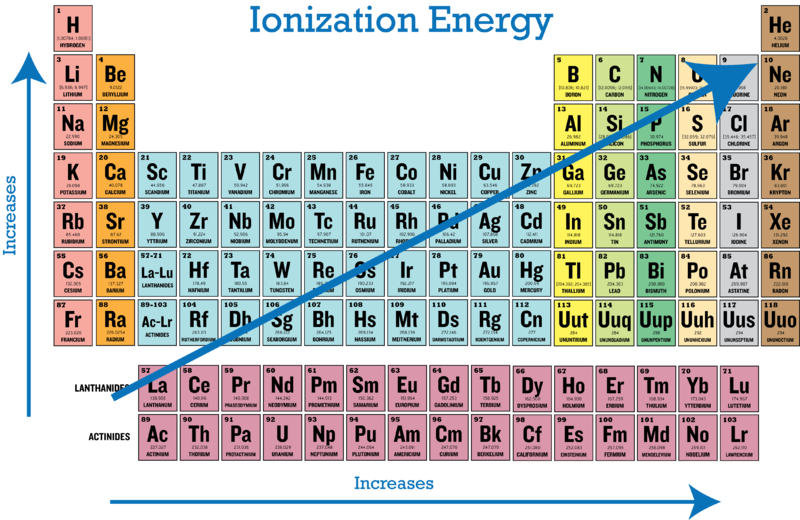
Periodic Trends In Ionization Energy Ck 12 Foundation

Which Element Has The Lowest First Ionization Energy Quora
Ionization Energy And Electron Affinity

Group Element 14 Carbon Family Properties Trends Videos Examples
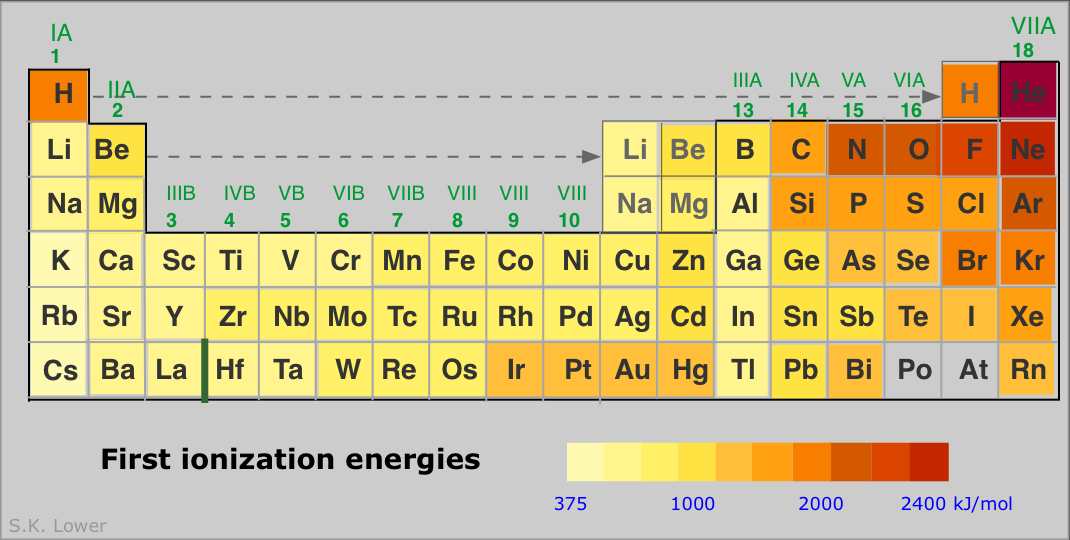
2 10 Periodic Properties Of The Elements Chemistry Libretexts



Comments
Post a Comment